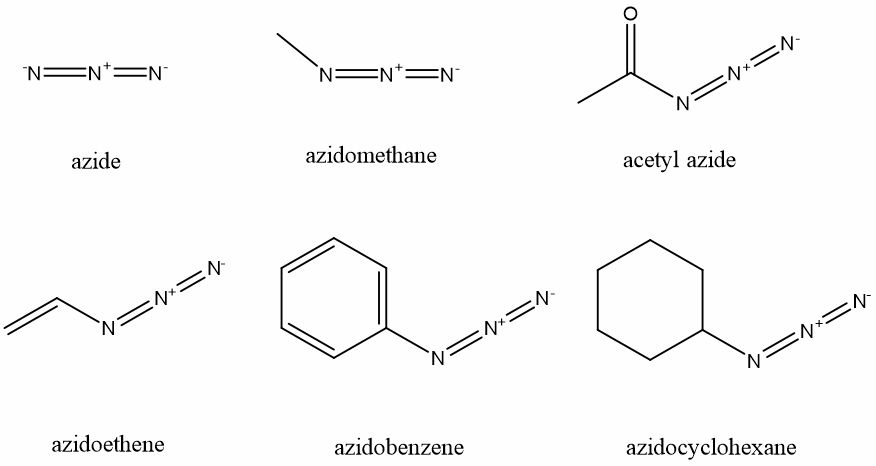
Azido impuriteis compounds have an azide functional group, which is linear, polyatomic anion consisting of three nitrogen atoms with the formula N3. Its structure can be represented as −N=N+=N−. Organic azides are organic compounds having the azido functional group attached to the organic compound (R) which is represented as RN3. This R may be alky, alkene, acetyl, aryl, cyclic or any other organic compound (Fig.1).
Azido impurities are mutagenic chemical substances that can impact genetic material through mutations. Long-term exposure can increase cancer risk. Although mutagenic, they pose a lower health risk compared to nitrosamines. Classifying them in Class 3 requires control at acceptable TTC limits. Identifying them at trace levels ensures drug safety.
The European Directorate for the Quality of Medicines & HealthCare (EDQM) reported in April 2021 about mutagenic azido impurities in angiotensin II receptor blockers (ARBs) and ‘Sartans’ class medicines, leading to the voluntary recall of hundreds of batches worldwide.
Azido impurities can be incorporated into drug substances and products through process formation, direct introduction, degradation, or cross-contamination, involving raw materials, intermediates, solvents, chemicals, and reagents in manufacturing.
The US Food and Drug Administration (FDA) has not issued guidelines on acceptable levels of azido impurities in pharmaceuticals. However, the European Medicines Agency (EMA) and the European Directorate for the Quality of Medicines (EDQM) have taken measures to prevent drug substances containing azido impurities from being released into the market. The Therapeutic Goods Administration (TGA) of Australia requires manufacturers to demonstrate their products are within acceptable limits for azido impurities before they can be approved for use. In Brazil, ANVISA requires pharmaceutical companies to conduct tests to identify and quantify azido impurities in their products and report any detected impurities to the agency. Health Canada holds manufacturers responsible for the safety and effectiveness of drugs sold in the Canadian market and has taken several actions to mitigate the risk.
Azido impurities are classified as Class-2 due to mutagenicity and unknown carcinogenicity data. Controlling them below the threshold of toxicological concern (TTC) is acceptable, with 1.5 μg per day intake acceptable for mutagenic impurities.
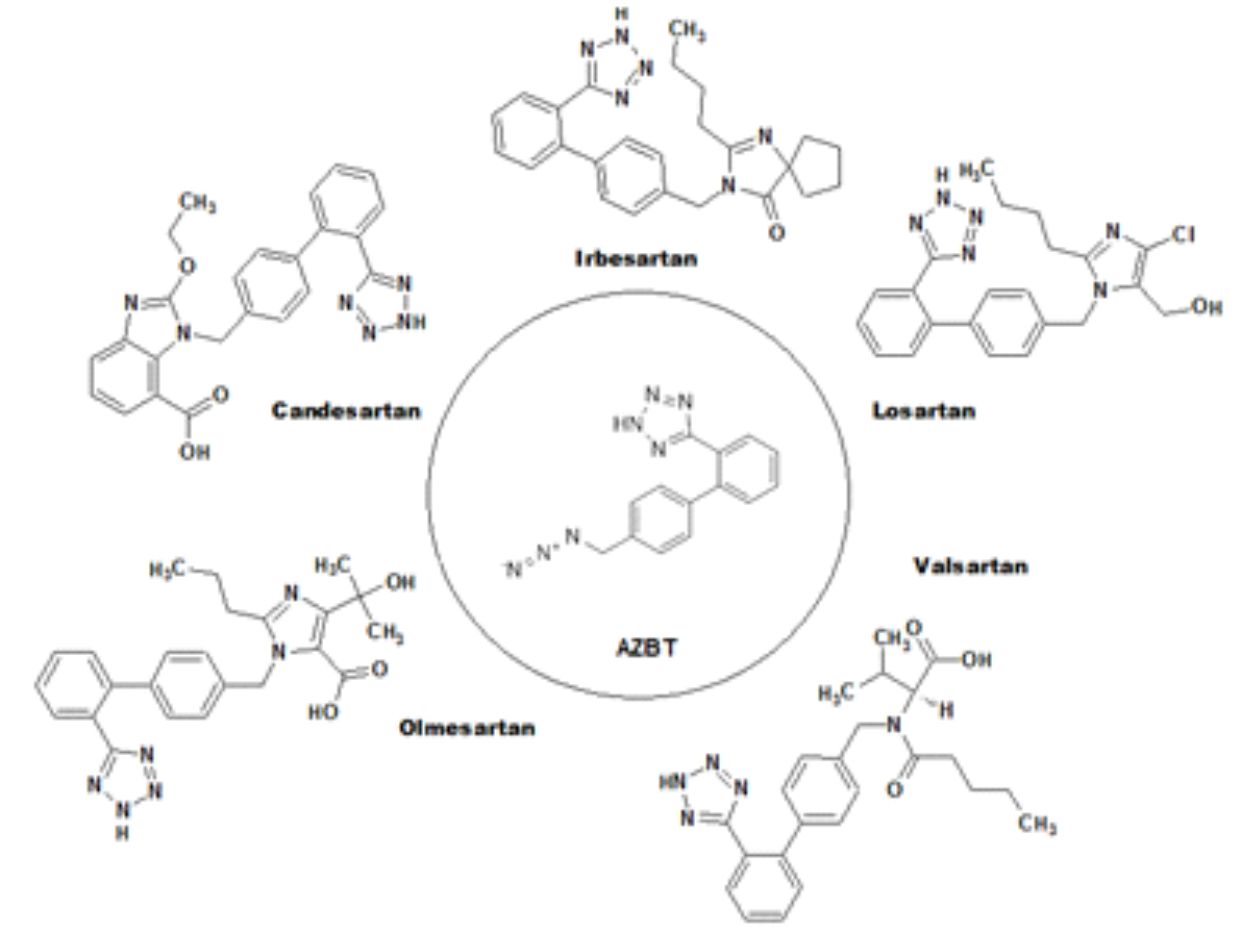
One of the azido impurities, 5-[4′-(Azidomethyl)[1,1′-biphenyl]-2-yl]-2H-tetrazole, also known as azidomethyl-biphenyl-tetrazole (AZBT), can form during the manufacturing of the active ingredient in some sartan drugs such as olmesartan, Losartan, Irbesartan, Valsartan, and Candesartan.
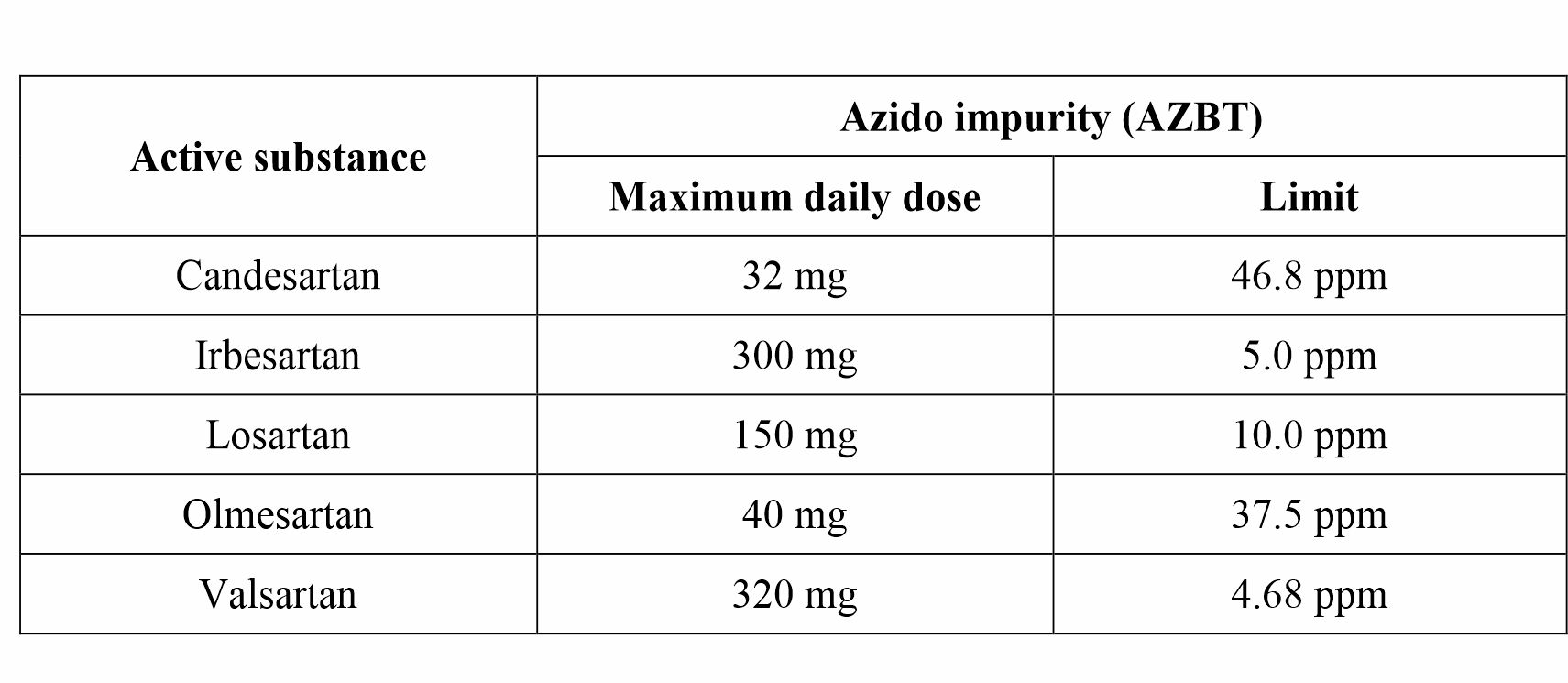
Considering the maximum daily doses for various sartan drugs, limits for azido impurity (AZBT) are calculated and tabulated below.
Limit for Azido impurity (AZBT) in sartans drugs
Analytical methods for determining azido impurities are challenging due to the low levels of impurities in complex matrices. Techniques like LCMS/MS or GCMS are used to detect and quantify these impurities. These methods must be validated to meet stringent regulatory requirements and conform to GMP requirements. Instrument manufacturers like Shimadzu, Thermo, Agilent, and Waters have published methods to cover various azido impurities in different ‘sartans’. Other regulatory authorities like Taiwan FDA and the German OMCL have also developed analytical methods for testing azido in drug substances and products.
Analytical method validation is the process of establishing documented evidence to ensure a specific procedure consistently produces a product meeting predetermined specifications and quality attributes. It is crucial for the quality of results and is essential for both qualitative and quantitative analytical methods. Method validation studies focus on quantitative analysis, particularly in impurity profiling, to ensure its suitability for its intended purpose.
References:
- European Directorate for the Quality of Medicines & Healthcare (2021, April 29). Risk of presence of mutagenic azido impurities in sartan active substances with a tetrazole ring, https://www.edqm.eu.
- European Directorate for the Quality of Medicines & Healthcare (2021, Sep. 29). Risk of the presence of mutagenic azido impurities in losartan active substance, https://www.edqm.eu
- Health Canada (2021, Oct. 26). Multiple lots of Irbesartan, losartan and valsartan drugs recalled, https://www.canada.ca/en/services/health.html.
- Teasdale, Andrew. “ICH M7: Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk.” ICH Quality Guidelines: An Implementation Guide (2017): 667-699.